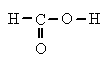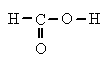formic acid or
methanoic acid
, HCO
2H, a colorless, corrosive liquid with a sharp odor; it boils at
100.7°C and solidifies at 8.4°C. It has the lowest molecular weight and is the
simplest of the carboxylic acids. Functionally, it is both an acid and an
aldehyde. Like other acids, it reacts with most alcohols to form esters and
decomposes when heated; like other aldehydes, it is easily oxidized. Formic acid
occurs in the bodies of red ants and in the stingers of bees. It can be made by
the oxidation of
formaldehyde; it is
prepared commercially by heating carbon monoxide and sodium hydroxide to form
sodium formate which, when carefully treated with sulfuric acid, yields formic
acid. Formic acid is used industrially in textile dyeing, in leather tanning,
and in coagulating latex rubber.
The Columbia Electronic Encyclopedia Copyright c 1994, 2000, Columbia University Press. Licensed from Columbia University Press. All rights reserved.